
Conjugation and Cross conjugation
Presence of alternate double bonds in a molecule is considered as conjugation.The molecule is said to be conjugated.
For example 1,3-Butadiene is a conjugated diene while 1,4-Pentadiene is not conjugated though it has two double bonds.

Conjugated diene, all carbons are sp2 hybridised. A non conjugated diene The 3rd carbon is sp3 hybridised
- In 1, 3-Butadiene all carbon atoms are sp2hybridised.
- Hence they all have a p-orbital each and they are parallel to each other. p-orbitals on adjacent atoms overlap permitting electron movement from one to the other. In 1, 3-Butadiene an electron from one end of the molecule can move to the other end through the p-orbital pathway.
- This movement of electrons through the p-orbital pathway is one more way of describing conjugation.
- In 1,4-Pentadiene the carbon in the centre does not have a p-orbital therefore movement of electrons is not possible from one end of the molecule to the other. Hence it is not possible to draw resonance structures.
- In the following orbital pictures the p-orbitals on adjacent atoms have been separated, but the understanding is that they overlap making electron movement possible. This is done so that the picture does not become confusing.
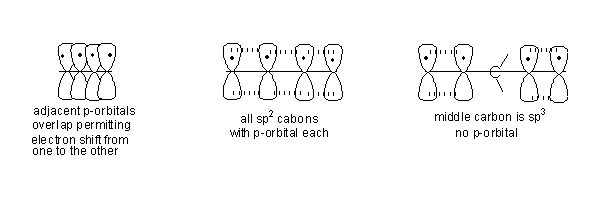
- A more conjugated compound absorbs light at higher λmax in the UV-VIS range.
- As conjugation increases λmaxincreases, this is because in a more conjugated compound the energy required for transition of the electron from Highest Occupied molecular Orbital (HOMO) to the Lowest Unoccupied Molecular orbital (LUMO) decreases hence the wavelength of maximum absorption increases.
- Benzene does not absorbs in the UV range only hence it is colourless to the human eye.
- Nitrobenzene has one more double bond in conjugation and its λmax increases and falls in the Visible range and we are able to see it as an yellow coloured substance.
Influence of conjugation on structure: See in the topic of resonance.
Copyrights: 2005 www.chemvista.org All Rights Reserved